Introduction:
According to the National Comprehensive Cancer Network (NCCN) 2019 guidelines on multiple myeloma (MM) treatment, three-drug regimens using bortezomib (V) and dexamethasone (d) in combination with either lenalidomide (R), (VRd) or Cyclophosphamide (C) combination (VCd) are standard first-line treatment options in newly diagnosed multiple myeloma (NDMM), regardless of transplant eligibility. However, the two combinations have not been studied head-to-head. This systemic review aims to compare the efficacy and safety outcomes of VCd versus VRd in NDMM patients.
Methods:
We performed a comprehensive literature search on PubMed, Cochrane Library, and ClinicalTrials.gov on July 27, 2020. We used the MeSH terms for 'multiple myeloma', 'cyclophosphamide', 'bortezomib', 'dexamethasone', 'lenalidomide', and their associated keywords. The search yielded 410 articles. Screening and data extraction were done according to PRISMA guidelines. After excluding case reports, case series, observational studies, review articles, meta-analysis, and retrospective studies, 11 phase II and III clinical trials (n=1088) were included of which three assessed VCd and eight assessed VRd in NDMM.
Results:
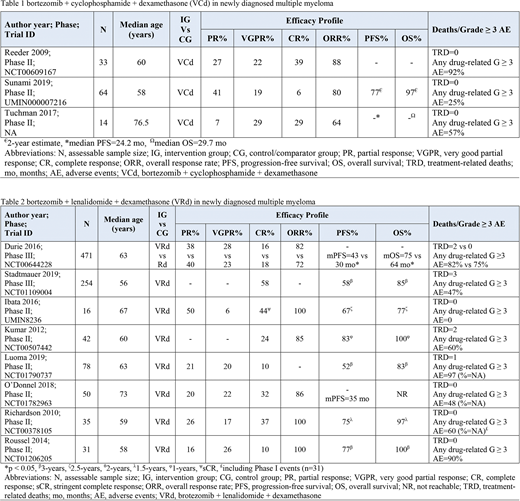
There was no trial on the direct comparison of VCd with VRd. Total 111 NDMM patients were evaluable who received VCd (Table 1). Moreover, total 977 NDMM patients were evaluable who received VRd (Table 2).
VCd arm results:
Reeder et al. (2009, n=33) achieved the best ORR of 88% which was largely VGPR or better (61%). Tuchman et al. (2017, n=14) reported similar results of ORR (64%) with 58% achieving VGPR or better and 7% achieving PR. Contrary to these ORR distributions, Sunami et al. (2019, n=64) reported the ORR of 80% which was largely driven by PR of 41%. Moreover, a 2-year OS of 97% and PFS of 77% which is consistent with previous results. The toxicity profile with VCd was manageable with mostly hematological adverse events. Neuropathy was significantly lower when compared to VRd.
VRd arm results:
NDMM patients, in a phase III trial by Durie et al. (2016, n=471) achieved ORR of 82% (PR=38%, VGPR=28%, and CR=16%) with VRd. The mPFS was 43 months and mOS was 75 months, both significantly better than control. Stadtmauer et al. (2019, n=254) results were similar as well with 3-year PFS and OS as 58% and 85% respectively. Kumar et al. (2012, n=42) had a 1-year OS of 100% and PFS of 83%. O'Donnel et al. (2018, n=50) achieved mPFS of 35 months while mOS could not be reached. Luoma et al. (2019, n=78) achieved a 3-year PFS of 52% while OS of 83%. Severe neuropathy was reported with VRd regimen. The remaining toxicity profile was mostly hematological and manageable with prompt intervention.
Conclusion:
The two upfront regimens of VCd and VRd show favorable efficacy and safety outcomes in NDMM patients. VRd arm achieves relatively higher rates of ORR, CR and VGPR. Cost-effectiveness and lower neuropathy incidence associated with VCd makes it favorable over VRd.
Anwer:Incyte, Seattle Genetics, Acetylon Pharmaceuticals, AbbVie Pharma, Astellas Pharma, Celegene, Millennium Pharmaceuticals.: Honoraria, Research Funding, Speakers Bureau.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal